I’ve touched on this tangentially in other posts, but now I want to focus properly on a pervasive misconception: that we do a stepped wedge trial because we want all of the sites (clusters) in our cluster-randomised trial to end up getting the intervention.
An ethical obligation?
First let me point out something important: if you’re convinced you have an ethical obligation to give everyone the intervention, and if there’s nothing stopping you, then that’s what you should do. Now. Right away. It’s as unethical to randomly allocate some clusters to wait a bit longer than others for the intervention as it would be to randomly allocate some clusters to continue to receive routine care for the duration of the trial (see Stepped wedge cluster randomised trials raise important ethical issues).
Remember that in a repeated cross-section or continuous recruitment stepped wedge design (see Three kinds of stepped wedge trial) each participant is assessed just once – either under the control condition (routine care) or under the intervention condition. During most stepped wedge trials of this kind half the participants don’t get the intervention. You need to be in some sort of equipoise to do this.
Of course, there might be practical constraints that mean you have to implement the intervention one cluster at a time, even if you would like to do it more quickly. But then the motivation for choosing a stepped wedge design is practical, not ethical (see Reasons for doing a stepped wedge trial).
A simple idea
Here’s a simple approach that can be used in any trial to ensure that all the sites end up getting the intervention: when the trial has finished, give all the sites the intervention.
Consider the design I gave as an example in Reasons for doing a stepped wedge trial, in which five of ten maternity units remain in the control condition for the duration of an 11-month trial, while the other five units are in the intervention condition for 11 months. If, after 11 months, we then offer the intervention to all of the units, we have a design that looks like this (dark green representing the intervention condition, light green the control):
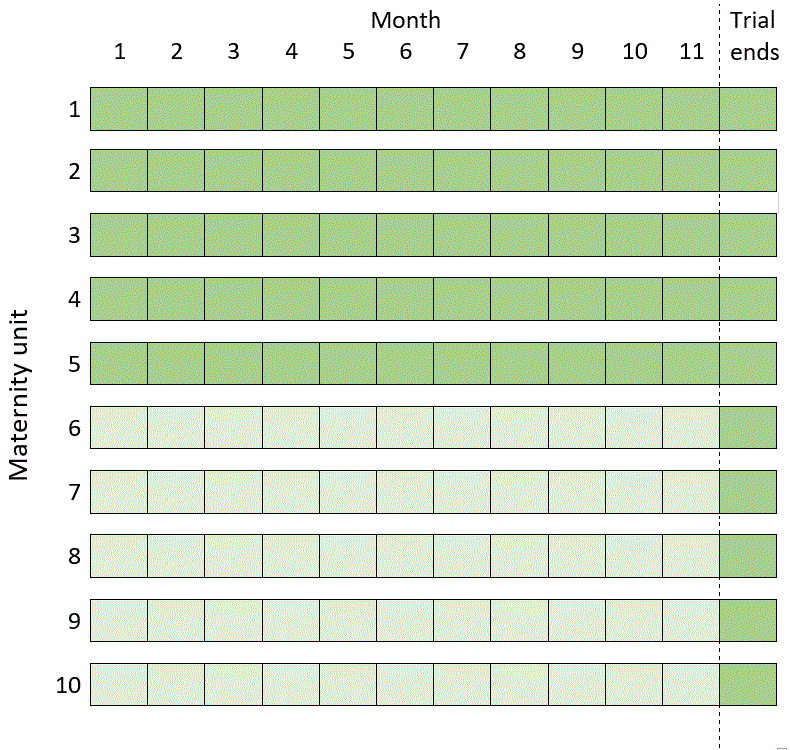
Incidentally, this is not quite the same thing as doing a 12-month trial in which all the units are crossed over to the intervention in the twelfth month. For one thing, the above design means that in month 12 we can get on with analysing the results from our 11-month study even while some units are still implementing the intervention. For another, it means that units can make up their own minds in month 12: they’re not obliged to take up the intervention if they don’t want to (our trial is finished, so compliance with a pre-determined schedule is no longer an issue).
Some investigators feel strongly that offering their novel intervention to all participating clusters will be an incentive to clusters to take part in the trial. It may well be, and in the example above, those ten maternity units that had agreed to participate in the trial could be offered the intervention preferentially in month 12, ahead of any wider roll-out to other units – but this doesn’t need to be part of the trial itself.
Of course, if you do want to extend the design so that for the last part of the trial proper, all the units are in the intervention condition, then that’s fine, but note that the example above (where there are just two distinct sequences to which clusters are randomised) is still too simple to be described as a stepped wedge (see What is a stepped wedge trial) – it’s more of a waiting-list control design where clusters, rather than individuals, are on a waiting list to receive the intervention.

Keeping it simple
I said in Reasons for doing a stepped wedge trial that when you’re considering a stepped wedge design it’s important to ask yourself how long you can reasonably ask any site to wait for the intervention. Suppose you’re planning a trial in 10 clusters and you don’t think you can reasonably ask any of them to wait longer than 11 months before implementing the intervention as part of the trial. Perhaps by then you fear that the intervention will start to become readily available to any cluster that goes out looking for it, compromising a properly randomised evaluation.
If you’re OK with the idea of evaluating these 10 clusters for an 11-month period, and if you don’t feel obliged to cross them over to the intervention during this time, then wouldn’t you consider randomising five of them to have the intervention throughout, while the other five remain in the control condition throughout? What are the thinking patterns that lead us to jump straight to the conclusion that this situation requires a stepped wedge trial?
Don’t get me wrong – I think that a stepped wedge design could turn out to be a good choice here, particularly for reasons of practicality or efficiency (see Reasons for doing a stepped wedge trial). I just want to try to break (and rebuild) some of the mental connections that get us to that point. A stepped wedge design certainly isn’t the simplest choice.
My conclusion
You want everyone to have the intervention? You don’t need a stepped wedge trial for that.
I think for individually randomised trials (complexs/behavioural interventions) there is a fair amount of evidence that waiting-list control groups are generally a bad idea because they lead to inflated treatment effect estimates. I’m not sure whether anybody investigated this for cluster randomised trials but if the same is true for these, wouldn’t this mean that a SWT would be preferable to your simple design because the inflation could be better accounted for?
Yes, you mean because the controls are holding back in anticipation of getting the intervention? I hadn’t really considered this – but then it seems to me that the same thing would apply in a stepped wedge trial: I’m not sure how this would better account for the inflated effect. Like you, I don’t know if anyone has investigated this.